james bond
Gold Member
- Oct 17, 2015
- 13,407
- 1,802
- 170
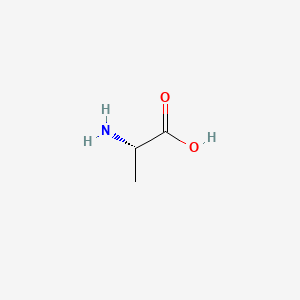
I think at this I will wrap it up with the icons of evolution argument presented by Jonathan Wells, an ID proponent, to strictly deride evolutionists (including God believers) and their thinking that life began from abiogenesis instead of Genesis. I presented his short video earlier.
Now, I present a lengthier discussion presented by Dr. Wells.
Now, I present a lengthier discussion presented by Dr. Wells.