Hollie - please note that I am posting actual scientific evidence - not merely rhetoric. You brought up the Miller-Urey experiment - I am simply posting the details of the results of that and similar origin of life synthesis experiments. Do you deny formic acid is the primary chemical reaction product? What chemical reaction product proportions have you found in your scientific research?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Does God Exist?
- Thread starter Bruce Daniels
- Start date
I didn't bring up Miller-Urey.Hollie - please note that I am posting actual scientific evidence - not merely rhetoric. You brought up the Miller-Urey experiment - I am simply posting the details of the results of that and similar origin of life synthesis experiments. Do you deny formic acid is the primary chemical reaction product? What chemical reaction product proportions have you found in your scientific research?
I don’t believe I am. But yes complexity has increased exponentially and did so according to the laws of nature. everything that has happened did so through cause and effect according to rules. These things are not random. The simple equation you provided is a rule which is effectively a cause which produces an effect.I think you are confusing complexity with intelligence.The intelligence is in the laws of nature which predestined those things. It’s not an accident that life and intelligence is programmed into the fabric of existence.
It is one of the most amazing discoveries in the realm of mathematics that not only does the simple equation Zn+1 = Zn2 + C create the infinitely complex Mandelbrot Set, but we can also find the same iconic shape in the patterns created by many other equations.

fncceo
Diamond Member
- Nov 29, 2016
- 43,150
- 35,916
- 3,615
They didn't even know why it rained.
Because I just washed my car.
Which part did I get wrong?The intelligence is in the laws of nature which predestined those things. It’s not an accident that life and intelligence is programmed into the fabric of existence.You think intelligence developed on earth before man? Zero evidence for that I'd say.Well we don’t know this is the first occurrence, right?
Do you really believe the organic micro machines of living organisms are happenstance? Have you seen the animations of the assembly line like machinations of these organic machines?
Yes I do. Simple natural laws can give rise to amazing things. Picture trillions of mindless molecules floating in water, what are the odds that they will form themselves into a regular geometric solid where every one is situated exactly like every other? Happens all the time, they are called crystals. No intelligence required.
You are only partly correct - normal for us humans, btw.
The fine tuned laws and properties of our universe allow for the creation of life and for intelligent life - however these do not evolve by chance.
For example, the precisely fine tuned rate for the expansion of our universe allowed for stars including supernovae to exist - and for supernovae to produce the elements needed for the creation of life.
Also the properties of these elements and complex compounds/molecules of these elements allow them to be arranged as informational rather than simply statistical molecules - for example: informational molecules (which also require translation and messenger molecules (e.g. messenger RNA).
However, information does not occur in molecules by chance - entropy works in the opposite direction - hence the difference between dead molecules and living molecules - at death information decays or leaves so that the functions of life cannot proceed.
For life to come into existence, informational molecules not only need to be created, along with translator molecules - but they need to be in the same place at the same time!
Btw - crystals are repetitive while informational molecules are variant.
The difficulty in creating life (which human creators cannot do) is illustrated in the environments needed to synthesize all of the 20 amino acids required for life:
Some amino acids prefer hot, others prefer cold for synthesis. Some prefer acid, others neutral or alkaline. Some prefer wet, others prefer dry - some even require condensing agents. You cannot have hot & cold, acid and alkaline, wet and dry in the same place at the same time. Unless, of course, an intelligent chemist is involved - of superior intelligence to us humans.
Would you all like me to post details as to the results of synthesis experiments like those of Miller - Urey, etc.? Suffice it to say for now that most are unaware that the primary chemical reaction product is formic acid, not amino acids. And that most amino acids and other molecules produced (the chemical reaction product proportions) are mostly useless (or worse) to life.
And, finally, chance synthesis of polypeptides from these amino acids and then further to proteins are always statistical, not informational. [chance formation of even statistical proteins has an incredibly low probability given favorable primordial soups.]
I should add the need for exact 3-d fit of enzymes and receptors for the life processes to proceed.
I’m sure you believe you did, JB.Trying to educate you on science is a waste of time.You do realize that matter and energy are equivalent, right?
No. Is a dingbat the equivalent of ding?
You can create matter, but not energy.
So whether I say matter or energy can’t exist without creating space time it like saying the same thing, right.
I think you're confused. People may think matter and energy are the same because they are found together. I agree both need space time. But then you can't create energy. Thus, big bang is impossible.
So what did God do?
so you understand the concept of space but are confused by space time. Space is the 3 dimensions represented by x, y and z coordinates. The 4th dimension t or time represents or is a measurement of expansion. Time doesn’t really exist it’s just a convenient way of demarcating the expansion of space. Space time is usually shown as a cone since the universe is relatively flat.
I’m not your enemy. So stop acting like I am.
C'mon you're just making stuff up. d = r * t, so t = d/r.
You fly from SF to NYC in a jet and your watch shows it takes you almost 6 hours. I go in the transporter and my watch shows less than a second has elapsed. Time exists and it's not always based on space expansion. Are you just saying that to fit big bang? What are you missing? Hint: Einstein.
Are you really an engineer?
And why does my post make you think I am your enemy?
I just debunked your space and time definition. It's not just the light cone.
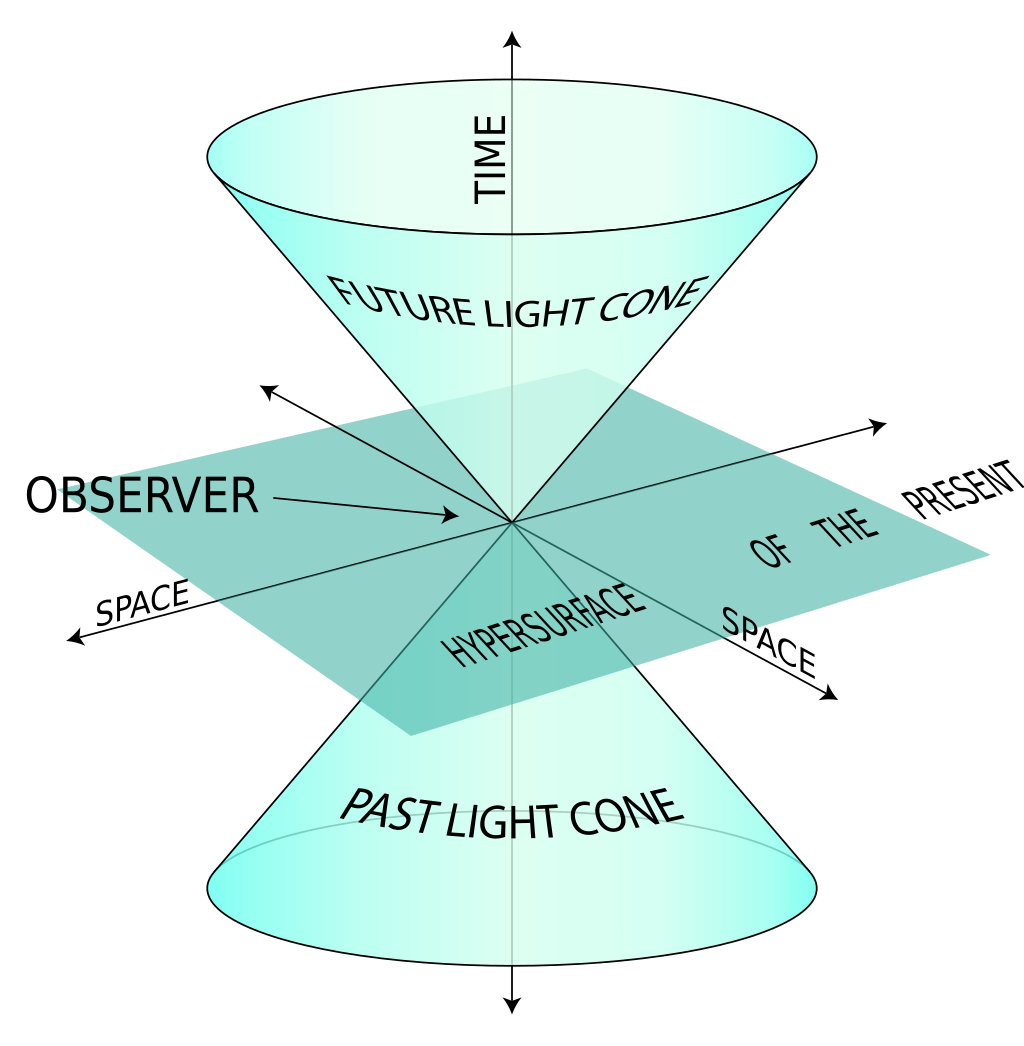
And God creating light first and space and time from the void is backed up by science while you just have a scrunched up face.
OK - from page 49, 50 from the above link:
".... many recent scenarios give HCN a prominent place. Also, a variety of HCN-derived nitriles have been suggested as having an important role as condensing agents in the synthesis of biologically significant polymers.34 Examples of condensing agents include cyanogen, cyanamide, dicyanamide, and cyanoacetylene. Some of these were mentioned in the review of ocean experiments in Chapter 3. The ease with which these cyano compounds enter into reaction with water is, however, a major barrier to their usefulness in synthesis. It is, of course, the ability of these cyano compounds to react with water that makes them attractive candidates as condensing agents. The role of a condensing agent is to remove the water that is split-out or produced as a byproduct in polymer formation. For example, when two amino acids react to form a dipeptide, a water molecule is released. Although dimer formation is thermodynamically unfavorable it can be made favorable simply by removing the water; hence the value of the condensing agent. A water solution, however, is a poor place for a condensing agent to perform its role. The condensing agent simply has no ability to discriminate between water molecules and will react with water from any source. Fig. 4-2 shows a number of the reactions to be expected in the primitive ocean."
Note that I earlier had noted that some amino acids prefer a dry, even with condensing agents, environment. (Others prefer wet, acid, alkaline, hot, cold)
Figure 4-2 shows a number of chemical reaction pathways that result from HCN in the presence of water - reacting with water: (= hydrolysis)
1. HCN to dicyanamide + H20 to cyanourea + H2O to Biuret
2. HCN to cyanomide & carbodiimide + H2O to Urea.
3. HCN to cyanogen +H2O to cyanoformamide + H2O to Ethanediamide.
4. HCN to cyanoacetyline + H2O to cyanoacetaldehyde
5. HCN + NH3 (ammonia) to formamidine + H20 to Formic acid
6. HCN + H2O to formamide + H2O to formic acid
All of these products (I have already noted formic acid) are useless to chemical reaction pathways to amino acids.
I will continue to detail the evidence once someone actually addresses the evidence instead of falsely stating that there is no evidence.
".... many recent scenarios give HCN a prominent place. Also, a variety of HCN-derived nitriles have been suggested as having an important role as condensing agents in the synthesis of biologically significant polymers.34 Examples of condensing agents include cyanogen, cyanamide, dicyanamide, and cyanoacetylene. Some of these were mentioned in the review of ocean experiments in Chapter 3. The ease with which these cyano compounds enter into reaction with water is, however, a major barrier to their usefulness in synthesis. It is, of course, the ability of these cyano compounds to react with water that makes them attractive candidates as condensing agents. The role of a condensing agent is to remove the water that is split-out or produced as a byproduct in polymer formation. For example, when two amino acids react to form a dipeptide, a water molecule is released. Although dimer formation is thermodynamically unfavorable it can be made favorable simply by removing the water; hence the value of the condensing agent. A water solution, however, is a poor place for a condensing agent to perform its role. The condensing agent simply has no ability to discriminate between water molecules and will react with water from any source. Fig. 4-2 shows a number of the reactions to be expected in the primitive ocean."
Note that I earlier had noted that some amino acids prefer a dry, even with condensing agents, environment. (Others prefer wet, acid, alkaline, hot, cold)
Figure 4-2 shows a number of chemical reaction pathways that result from HCN in the presence of water - reacting with water: (= hydrolysis)
1. HCN to dicyanamide + H20 to cyanourea + H2O to Biuret
2. HCN to cyanomide & carbodiimide + H2O to Urea.
3. HCN to cyanogen +H2O to cyanoformamide + H2O to Ethanediamide.
4. HCN to cyanoacetyline + H2O to cyanoacetaldehyde
5. HCN + NH3 (ammonia) to formamidine + H20 to Formic acid
6. HCN + H2O to formamide + H2O to formic acid
All of these products (I have already noted formic acid) are useless to chemical reaction pathways to amino acids.
I will continue to detail the evidence once someone actually addresses the evidence instead of falsely stating that there is no evidence.
The obvious problem with the book you linked to is the presumption that the authors have a predefined conclusion.T
We can only speculate on what the conditions of the early earth were and what the first life was made of. Here is some on the carbon cycle. I do know that there was plenty of oxygen around, I'm pretty sure it was not free, atmospheric oxygen, at least not for long.
The only evidence for an intelligent chemist is a gap in our knowledge. Such gaps have been getting smaller and smaller as we learn more.
Go ahead and add the early gases which you claim. What happens to the Miller-Urey experiment?
Miller-Urey Experiment
www.millerureyexperiment.com
Thaxton, et al, documented the actual chemical reaction product proportions in this book:
From page 23:
"In 1974, Miller reported the amino acids he had obtained in electrical discharge experiments.8 These are listed in table 3-2."
Reference 8. Miller and Orgel, The Origins of Life on the Earth, p. 84. See also: D. Ring, Y. Wolman, N. Friedmann, and S. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 765; Y. Wolman, W.J. Haverland, and S.L. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 809; S. Miller, 1955. J. Am. Chem. Soc. 77,2351.
Table3-2. Yields of amino acids obtained from sparking a mixture of CFL, NHl, H20 and H2.
Compound
Glycine Alanine a-Amino-n-butyric acid a-Aminoisobutyric acid Valine Norvaline Isovaline Leucine Isoleucine Alloisoleucine Norleucine tert-Leucine Proline Aspartic acid Glutamic acid Serine Threonine Allothreonine
Yield (14M) Compound Yield (14M)
440 790 270 30 19.5 61 5 11.3 4.8 5.1 6.0 < 0.02 1.5 34 7.7 5.0 0.8 0.8
a:y-Diaminobutyric acid a-Hydroxy-')'-aminobutyric acid Sarcosine N-Ethylglycine N-Propylglycine N-Isopropylglycine N-Methylalanine N-Ethylalanine ,8-Alanine ,8-Amino-n-butyric acid ,8-Amino-isobutyric acid "Y-Aminobutyric acid N-Methyl-,8-alanine N-Ethyl-,8-alanine Pipecolic acid a,,B-Diaminopropionic acid Isoserine
33 74 55 30 2 2 15 < 0.2 18.8 0.3 0.3 2.4 5 2 0.05 6.4 5.5
(From S. Miller, 1974. Origins of Life 5, 139.)
See the link for correct formatting.
Note this list does not include formic acid, the primary product, because it is not an amino acid.
Note Glycine has the highest proportion. This is because Glycine is the simplest amino acid. From Bing search:
"The simplest, and smallest, amino acid found in proteins is glycine for which the R-group is a hydrogen (H)."
There are about 500 naturally occurring amino acids - only 20 (L polarized) are used in life.
Table 3-3 (page 25) includes the primary chemical reaction product: formic acid. It is the base of comparison of chemical reaction product proportions with a number of 1,000. By comparison, Glycine is 270 and Glycolic acid is 240. Some of the amino acids produced are extremely low percentage compared with non-biologic product proportions - see the chart.
See the rest of the evidence of different synthesis experiments in different environments in documented in chapter 3.
Chapter 4 is entitled "The myth of of the prebiotic soup" shows much evidence from chemistry.
I will post just one example in this post. From page 48:[HCN is Hydrogen Cyanide]
"Hydrolysis of HCN and Nitriles (RCN)
According to Ponnamperuma, hydrogen cyanide may be "the most important intermediate leading to the origin of life."29 It is an ingredient for the production of amino acids in the Strecker synthesis (see Chapter 3). It also is considered a starting material in the synthesis of adenine and a host of other biomolecules, as shown in figure 4-1. The value of HCN in the chemical evolution scenario is enhanced by the fact that it escapes rapid destruction in the atmosphere by ultraviolet irradiation.30
....
It is the ubiquitous water molecule, however, that is the main obstacle to the reaction involving HCN and its nitrile derivatives.31 For example, HCN adds water to its triple bond to form formamide, which, upon further hydrolysis, produces formic acid."
This is why formic acid, not amino acids. is the primary chemical reaction product in synthesis experiments including Miller-Urey.
This is an example of basic chemistry ignored by chemical evolutionists. Simply: HCN + H2O yields formamide + H2O yields formic acid.
Page 50 goes into more detail as to the destruction of chemical pathways to molecules required for life.
That is for my next post.
Charles Thaxton is affiliated with charlatans at the Disco'tute. Dean Kenyon is a notorious YEC'ist who is credible only among like-minded charlatans.
I didn't bring up Miller-Urey.Hollie - please note that I am posting actual scientific evidence - not merely rhetoric. You brought up the Miller-Urey experiment - I am simply posting the details of the results of that and similar origin of life synthesis experiments. Do you deny formic acid is the primary chemical reaction product? What chemical reaction product proportions have you found in your scientific research?
Yes you did - but apparently you deleted your post. Unless I am going nuts!
My point is that the actual evidence from chemistry is ignored by chemical evolutionists - not to mention almost everyone else! Even our literature does not mention the hydrolysis of HCN! But HCN is useless to origin of life chemical pathways if water is present - that is a basic fact of chemistry which I have detailed.
The obvious problem with the book you linked to is the presumption that the authors have a predefined conclusion.T
We can only speculate on what the conditions of the early earth were and what the first life was made of. Here is some on the carbon cycle. I do know that there was plenty of oxygen around, I'm pretty sure it was not free, atmospheric oxygen, at least not for long.
The only evidence for an intelligent chemist is a gap in our knowledge. Such gaps have been getting smaller and smaller as we learn more.
Go ahead and add the early gases which you claim. What happens to the Miller-Urey experiment?
Miller-Urey Experiment
www.millerureyexperiment.com
Thaxton, et al, documented the actual chemical reaction product proportions in this book:
From page 23:
"In 1974, Miller reported the amino acids he had obtained in electrical discharge experiments.8 These are listed in table 3-2."
Reference 8. Miller and Orgel, The Origins of Life on the Earth, p. 84. See also: D. Ring, Y. Wolman, N. Friedmann, and S. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 765; Y. Wolman, W.J. Haverland, and S.L. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 809; S. Miller, 1955. J. Am. Chem. Soc. 77,2351.
Table3-2. Yields of amino acids obtained from sparking a mixture of CFL, NHl, H20 and H2.
Compound
Glycine Alanine a-Amino-n-butyric acid a-Aminoisobutyric acid Valine Norvaline Isovaline Leucine Isoleucine Alloisoleucine Norleucine tert-Leucine Proline Aspartic acid Glutamic acid Serine Threonine Allothreonine
Yield (14M) Compound Yield (14M)
440 790 270 30 19.5 61 5 11.3 4.8 5.1 6.0 < 0.02 1.5 34 7.7 5.0 0.8 0.8
a:y-Diaminobutyric acid a-Hydroxy-')'-aminobutyric acid Sarcosine N-Ethylglycine N-Propylglycine N-Isopropylglycine N-Methylalanine N-Ethylalanine ,8-Alanine ,8-Amino-n-butyric acid ,8-Amino-isobutyric acid "Y-Aminobutyric acid N-Methyl-,8-alanine N-Ethyl-,8-alanine Pipecolic acid a,,B-Diaminopropionic acid Isoserine
33 74 55 30 2 2 15 < 0.2 18.8 0.3 0.3 2.4 5 2 0.05 6.4 5.5
(From S. Miller, 1974. Origins of Life 5, 139.)
See the link for correct formatting.
Note this list does not include formic acid, the primary product, because it is not an amino acid.
Note Glycine has the highest proportion. This is because Glycine is the simplest amino acid. From Bing search:
"The simplest, and smallest, amino acid found in proteins is glycine for which the R-group is a hydrogen (H)."
There are about 500 naturally occurring amino acids - only 20 (L polarized) are used in life.
Table 3-3 (page 25) includes the primary chemical reaction product: formic acid. It is the base of comparison of chemical reaction product proportions with a number of 1,000. By comparison, Glycine is 270 and Glycolic acid is 240. Some of the amino acids produced are extremely low percentage compared with non-biologic product proportions - see the chart.
See the rest of the evidence of different synthesis experiments in different environments in documented in chapter 3.
Chapter 4 is entitled "The myth of of the prebiotic soup" shows much evidence from chemistry.
I will post just one example in this post. From page 48:[HCN is Hydrogen Cyanide]
"Hydrolysis of HCN and Nitriles (RCN)
According to Ponnamperuma, hydrogen cyanide may be "the most important intermediate leading to the origin of life."29 It is an ingredient for the production of amino acids in the Strecker synthesis (see Chapter 3). It also is considered a starting material in the synthesis of adenine and a host of other biomolecules, as shown in figure 4-1. The value of HCN in the chemical evolution scenario is enhanced by the fact that it escapes rapid destruction in the atmosphere by ultraviolet irradiation.30
....
It is the ubiquitous water molecule, however, that is the main obstacle to the reaction involving HCN and its nitrile derivatives.31 For example, HCN adds water to its triple bond to form formamide, which, upon further hydrolysis, produces formic acid."
This is why formic acid, not amino acids. is the primary chemical reaction product in synthesis experiments including Miller-Urey.
This is an example of basic chemistry ignored by chemical evolutionists. Simply: HCN + H2O yields formamide + H2O yields formic acid.
Page 50 goes into more detail as to the destruction of chemical pathways to molecules required for life.
That is for my next post.
Charles Thaxton is affiliated with charlatans at the Disco'tute. Dean Kenyon is a notorious YEC'ist who is credible only among like-minded charlatans.
What are you claiming there is evidence for?OK - from page 49, 50 from the above link:
".... many recent scenarios give HCN a prominent place. Also, a variety of HCN-derived nitriles have been suggested as having an important role as condensing agents in the synthesis of biologically significant polymers.34 Examples of condensing agents include cyanogen, cyanamide, dicyanamide, and cyanoacetylene. Some of these were mentioned in the review of ocean experiments in Chapter 3. The ease with which these cyano compounds enter into reaction with water is, however, a major barrier to their usefulness in synthesis. It is, of course, the ability of these cyano compounds to react with water that makes them attractive candidates as condensing agents. The role of a condensing agent is to remove the water that is split-out or produced as a byproduct in polymer formation. For example, when two amino acids react to form a dipeptide, a water molecule is released. Although dimer formation is thermodynamically unfavorable it can be made favorable simply by removing the water; hence the value of the condensing agent. A water solution, however, is a poor place for a condensing agent to perform its role. The condensing agent simply has no ability to discriminate between water molecules and will react with water from any source. Fig. 4-2 shows a number of the reactions to be expected in the primitive ocean."
Note that I earlier had noted that some amino acids prefer a dry, even with condensing agents, environment. (Others prefer wet, acid, alkaline, hot, cold)
Figure 4-2 shows a number of chemical reaction pathways that result from HCN in the presence of water - reacting with water: (= hydrolysis)
1. HCN to dicyanamide + H20 to cyanourea + H2O to Biuret
2. HCN to cyanomide & carbodiimide + H2O to Urea.
3. HCN to cyanogen +H2O to cyanoformamide + H2O to Ethanediamide.
4. HCN to cyanoacetyline + H2O to cyanoacetaldehyde
5. HCN + NH3 (ammonia) to formamidine + H20 to Formic acid
6. HCN + H2O to formamide + H2O to formic acid
All of these products (I have already noted formic acid) are useless to chemical reaction pathways to amino acids.
I will continue to detail the evidence once someone actually addresses the evidence instead of falsely stating that there is no evidence.
I never brought up Miller-Urey.I didn't bring up Miller-Urey.Hollie - please note that I am posting actual scientific evidence - not merely rhetoric. You brought up the Miller-Urey experiment - I am simply posting the details of the results of that and similar origin of life synthesis experiments. Do you deny formic acid is the primary chemical reaction product? What chemical reaction product proportions have you found in your scientific research?
Yes you did - but apparently you deleted your post. Unless I am going nuts!
My point is that the actual evidence from chemistry is ignored by chemical evolutionists - not to mention almost everyone else! Even our literature does not mention the hydrolysis of HCN! But HCN is useless to origin of life chemical pathways if water is present - that is a basic fact of chemistry which I have detailed.
What is a chemical evolutionist?
A
A typical debate tactic on the incorrect side of a debate is to attack the person reporting the scientific evidence instead of actually addressing the evidence.
I doubt you will actually post about the chemical reaction of HCN with water - but feel free to surprise me!
Oh, and surprise me more by actually confirming the chemical reaction product proportions in origin of life synthesis experiments! Do you deny formic acid rather than amino acids is the predominant product?
The obvious problem with the book you linked to is the presumption that the authors have a predefined conclusion.T
We can only speculate on what the conditions of the early earth were and what the first life was made of. Here is some on the carbon cycle. I do know that there was plenty of oxygen around, I'm pretty sure it was not free, atmospheric oxygen, at least not for long.
The only evidence for an intelligent chemist is a gap in our knowledge. Such gaps have been getting smaller and smaller as we learn more.
Go ahead and add the early gases which you claim. What happens to the Miller-Urey experiment?
Miller-Urey Experiment
www.millerureyexperiment.com
Thaxton, et al, documented the actual chemical reaction product proportions in this book:
From page 23:
"In 1974, Miller reported the amino acids he had obtained in electrical discharge experiments.8 These are listed in table 3-2."
Reference 8. Miller and Orgel, The Origins of Life on the Earth, p. 84. See also: D. Ring, Y. Wolman, N. Friedmann, and S. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 765; Y. Wolman, W.J. Haverland, and S.L. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 809; S. Miller, 1955. J. Am. Chem. Soc. 77,2351.
Table3-2. Yields of amino acids obtained from sparking a mixture of CFL, NHl, H20 and H2.
Compound
Glycine Alanine a-Amino-n-butyric acid a-Aminoisobutyric acid Valine Norvaline Isovaline Leucine Isoleucine Alloisoleucine Norleucine tert-Leucine Proline Aspartic acid Glutamic acid Serine Threonine Allothreonine
Yield (14M) Compound Yield (14M)
440 790 270 30 19.5 61 5 11.3 4.8 5.1 6.0 < 0.02 1.5 34 7.7 5.0 0.8 0.8
a:y-Diaminobutyric acid a-Hydroxy-')'-aminobutyric acid Sarcosine N-Ethylglycine N-Propylglycine N-Isopropylglycine N-Methylalanine N-Ethylalanine ,8-Alanine ,8-Amino-n-butyric acid ,8-Amino-isobutyric acid "Y-Aminobutyric acid N-Methyl-,8-alanine N-Ethyl-,8-alanine Pipecolic acid a,,B-Diaminopropionic acid Isoserine
33 74 55 30 2 2 15 < 0.2 18.8 0.3 0.3 2.4 5 2 0.05 6.4 5.5
(From S. Miller, 1974. Origins of Life 5, 139.)
See the link for correct formatting.
Note this list does not include formic acid, the primary product, because it is not an amino acid.
Note Glycine has the highest proportion. This is because Glycine is the simplest amino acid. From Bing search:
"The simplest, and smallest, amino acid found in proteins is glycine for which the R-group is a hydrogen (H)."
There are about 500 naturally occurring amino acids - only 20 (L polarized) are used in life.
Table 3-3 (page 25) includes the primary chemical reaction product: formic acid. It is the base of comparison of chemical reaction product proportions with a number of 1,000. By comparison, Glycine is 270 and Glycolic acid is 240. Some of the amino acids produced are extremely low percentage compared with non-biologic product proportions - see the chart.
See the rest of the evidence of different synthesis experiments in different environments in documented in chapter 3.
Chapter 4 is entitled "The myth of of the prebiotic soup" shows much evidence from chemistry.
I will post just one example in this post. From page 48:[HCN is Hydrogen Cyanide]
"Hydrolysis of HCN and Nitriles (RCN)
According to Ponnamperuma, hydrogen cyanide may be "the most important intermediate leading to the origin of life."29 It is an ingredient for the production of amino acids in the Strecker synthesis (see Chapter 3). It also is considered a starting material in the synthesis of adenine and a host of other biomolecules, as shown in figure 4-1. The value of HCN in the chemical evolution scenario is enhanced by the fact that it escapes rapid destruction in the atmosphere by ultraviolet irradiation.30
....
It is the ubiquitous water molecule, however, that is the main obstacle to the reaction involving HCN and its nitrile derivatives.31 For example, HCN adds water to its triple bond to form formamide, which, upon further hydrolysis, produces formic acid."
This is why formic acid, not amino acids. is the primary chemical reaction product in synthesis experiments including Miller-Urey.
This is an example of basic chemistry ignored by chemical evolutionists. Simply: HCN + H2O yields formamide + H2O yields formic acid.
Page 50 goes into more detail as to the destruction of chemical pathways to molecules required for life.
That is for my next post.
Charles Thaxton is affiliated with charlatans at the Disco'tute. Dean Kenyon is a notorious YEC'ist who is credible only among like-minded charlatans.
A typical debate tactic on the incorrect side of a debate is to attack the person reporting the scientific evidence instead of actually addressing the evidence.
I doubt you will actually post about the chemical reaction of HCN with water - but feel free to surprise me!
Oh, and surprise me more by actually confirming the chemical reaction product proportions in origin of life synthesis experiments! Do you deny formic acid rather than amino acids is the predominant product?
The obvious problem with the book you linked to is the presumption that the authors have a predefined conclusion.T
We can only speculate on what the conditions of the early earth were and what the first life was made of. Here is some on the carbon cycle. I do know that there was plenty of oxygen around, I'm pretty sure it was not free, atmospheric oxygen, at least not for long.
The only evidence for an intelligent chemist is a gap in our knowledge. Such gaps have been getting smaller and smaller as we learn more.
Go ahead and add the early gases which you claim. What happens to the Miller-Urey experiment?
Miller-Urey Experiment
www.millerureyexperiment.com
Thaxton, et al, documented the actual chemical reaction product proportions in this book:
From page 23:
"In 1974, Miller reported the amino acids he had obtained in electrical discharge experiments.8 These are listed in table 3-2."
Reference 8. Miller and Orgel, The Origins of Life on the Earth, p. 84. See also: D. Ring, Y. Wolman, N. Friedmann, and S. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 765; Y. Wolman, W.J. Haverland, and S.L. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 809; S. Miller, 1955. J. Am. Chem. Soc. 77,2351.
Table3-2. Yields of amino acids obtained from sparking a mixture of CFL, NHl, H20 and H2.
Compound
Glycine Alanine a-Amino-n-butyric acid a-Aminoisobutyric acid Valine Norvaline Isovaline Leucine Isoleucine Alloisoleucine Norleucine tert-Leucine Proline Aspartic acid Glutamic acid Serine Threonine Allothreonine
Yield (14M) Compound Yield (14M)
440 790 270 30 19.5 61 5 11.3 4.8 5.1 6.0 < 0.02 1.5 34 7.7 5.0 0.8 0.8
a:y-Diaminobutyric acid a-Hydroxy-')'-aminobutyric acid Sarcosine N-Ethylglycine N-Propylglycine N-Isopropylglycine N-Methylalanine N-Ethylalanine ,8-Alanine ,8-Amino-n-butyric acid ,8-Amino-isobutyric acid "Y-Aminobutyric acid N-Methyl-,8-alanine N-Ethyl-,8-alanine Pipecolic acid a,,B-Diaminopropionic acid Isoserine
33 74 55 30 2 2 15 < 0.2 18.8 0.3 0.3 2.4 5 2 0.05 6.4 5.5
(From S. Miller, 1974. Origins of Life 5, 139.)
See the link for correct formatting.
Note this list does not include formic acid, the primary product, because it is not an amino acid.
Note Glycine has the highest proportion. This is because Glycine is the simplest amino acid. From Bing search:
"The simplest, and smallest, amino acid found in proteins is glycine for which the R-group is a hydrogen (H)."
There are about 500 naturally occurring amino acids - only 20 (L polarized) are used in life.
Table 3-3 (page 25) includes the primary chemical reaction product: formic acid. It is the base of comparison of chemical reaction product proportions with a number of 1,000. By comparison, Glycine is 270 and Glycolic acid is 240. Some of the amino acids produced are extremely low percentage compared with non-biologic product proportions - see the chart.
See the rest of the evidence of different synthesis experiments in different environments in documented in chapter 3.
Chapter 4 is entitled "The myth of of the prebiotic soup" shows much evidence from chemistry.
I will post just one example in this post. From page 48:[HCN is Hydrogen Cyanide]
"Hydrolysis of HCN and Nitriles (RCN)
According to Ponnamperuma, hydrogen cyanide may be "the most important intermediate leading to the origin of life."29 It is an ingredient for the production of amino acids in the Strecker synthesis (see Chapter 3). It also is considered a starting material in the synthesis of adenine and a host of other biomolecules, as shown in figure 4-1. The value of HCN in the chemical evolution scenario is enhanced by the fact that it escapes rapid destruction in the atmosphere by ultraviolet irradiation.30
....
It is the ubiquitous water molecule, however, that is the main obstacle to the reaction involving HCN and its nitrile derivatives.31 For example, HCN adds water to its triple bond to form formamide, which, upon further hydrolysis, produces formic acid."
This is why formic acid, not amino acids. is the primary chemical reaction product in synthesis experiments including Miller-Urey.
This is an example of basic chemistry ignored by chemical evolutionists. Simply: HCN + H2O yields formamide + H2O yields formic acid.
Page 50 goes into more detail as to the destruction of chemical pathways to molecules required for life.
That is for my next post.
Charles Thaxton is affiliated with charlatans at the Disco'tute. Dean Kenyon is a notorious YEC'ist who is credible only among like-minded charlatans.What are you claiming there is evidence for?OK - from page 49, 50 from the above link:
".... many recent scenarios give HCN a prominent place. Also, a variety of HCN-derived nitriles have been suggested as having an important role as condensing agents in the synthesis of biologically significant polymers.34 Examples of condensing agents include cyanogen, cyanamide, dicyanamide, and cyanoacetylene. Some of these were mentioned in the review of ocean experiments in Chapter 3. The ease with which these cyano compounds enter into reaction with water is, however, a major barrier to their usefulness in synthesis. It is, of course, the ability of these cyano compounds to react with water that makes them attractive candidates as condensing agents. The role of a condensing agent is to remove the water that is split-out or produced as a byproduct in polymer formation. For example, when two amino acids react to form a dipeptide, a water molecule is released. Although dimer formation is thermodynamically unfavorable it can be made favorable simply by removing the water; hence the value of the condensing agent. A water solution, however, is a poor place for a condensing agent to perform its role. The condensing agent simply has no ability to discriminate between water molecules and will react with water from any source. Fig. 4-2 shows a number of the reactions to be expected in the primitive ocean."
Note that I earlier had noted that some amino acids prefer a dry, even with condensing agents, environment. (Others prefer wet, acid, alkaline, hot, cold)
Figure 4-2 shows a number of chemical reaction pathways that result from HCN in the presence of water - reacting with water: (= hydrolysis)
1. HCN to dicyanamide + H20 to cyanourea + H2O to Biuret
2. HCN to cyanomide & carbodiimide + H2O to Urea.
3. HCN to cyanogen +H2O to cyanoformamide + H2O to Ethanediamide.
4. HCN to cyanoacetyline + H2O to cyanoacetaldehyde
5. HCN + NH3 (ammonia) to formamidine + H20 to Formic acid
6. HCN + H2O to formamide + H2O to formic acid
All of these products (I have already noted formic acid) are useless to chemical reaction pathways to amino acids.
I will continue to detail the evidence once someone actually addresses the evidence instead of falsely stating that there is no evidence.
Chemical reaction products - please read my posts more carefully.
The problem with your complaint is that you selectively post from sources you know have a bias toward the result you want. Your sources announce their bias toward a predisposed result so, yes, it is legitimate to identify their bias. That's not attacking your source but identifying fairly that your sources intend to present an argument that meets a predefined conclusion.A
The obvious problem with the book you linked to is the presumption that the authors have a predefined conclusion.T
We can only speculate on what the conditions of the early earth were and what the first life was made of. Here is some on the carbon cycle. I do know that there was plenty of oxygen around, I'm pretty sure it was not free, atmospheric oxygen, at least not for long.
The only evidence for an intelligent chemist is a gap in our knowledge. Such gaps have been getting smaller and smaller as we learn more.
Go ahead and add the early gases which you claim. What happens to the Miller-Urey experiment?
Miller-Urey Experiment
www.millerureyexperiment.com
Thaxton, et al, documented the actual chemical reaction product proportions in this book:
From page 23:
"In 1974, Miller reported the amino acids he had obtained in electrical discharge experiments.8 These are listed in table 3-2."
Reference 8. Miller and Orgel, The Origins of Life on the Earth, p. 84. See also: D. Ring, Y. Wolman, N. Friedmann, and S. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 765; Y. Wolman, W.J. Haverland, and S.L. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 809; S. Miller, 1955. J. Am. Chem. Soc. 77,2351.
Table3-2. Yields of amino acids obtained from sparking a mixture of CFL, NHl, H20 and H2.
Compound
Glycine Alanine a-Amino-n-butyric acid a-Aminoisobutyric acid Valine Norvaline Isovaline Leucine Isoleucine Alloisoleucine Norleucine tert-Leucine Proline Aspartic acid Glutamic acid Serine Threonine Allothreonine
Yield (14M) Compound Yield (14M)
440 790 270 30 19.5 61 5 11.3 4.8 5.1 6.0 < 0.02 1.5 34 7.7 5.0 0.8 0.8
a:y-Diaminobutyric acid a-Hydroxy-')'-aminobutyric acid Sarcosine N-Ethylglycine N-Propylglycine N-Isopropylglycine N-Methylalanine N-Ethylalanine ,8-Alanine ,8-Amino-n-butyric acid ,8-Amino-isobutyric acid "Y-Aminobutyric acid N-Methyl-,8-alanine N-Ethyl-,8-alanine Pipecolic acid a,,B-Diaminopropionic acid Isoserine
33 74 55 30 2 2 15 < 0.2 18.8 0.3 0.3 2.4 5 2 0.05 6.4 5.5
(From S. Miller, 1974. Origins of Life 5, 139.)
See the link for correct formatting.
Note this list does not include formic acid, the primary product, because it is not an amino acid.
Note Glycine has the highest proportion. This is because Glycine is the simplest amino acid. From Bing search:
"The simplest, and smallest, amino acid found in proteins is glycine for which the R-group is a hydrogen (H)."
There are about 500 naturally occurring amino acids - only 20 (L polarized) are used in life.
Table 3-3 (page 25) includes the primary chemical reaction product: formic acid. It is the base of comparison of chemical reaction product proportions with a number of 1,000. By comparison, Glycine is 270 and Glycolic acid is 240. Some of the amino acids produced are extremely low percentage compared with non-biologic product proportions - see the chart.
See the rest of the evidence of different synthesis experiments in different environments in documented in chapter 3.
Chapter 4 is entitled "The myth of of the prebiotic soup" shows much evidence from chemistry.
I will post just one example in this post. From page 48:[HCN is Hydrogen Cyanide]
"Hydrolysis of HCN and Nitriles (RCN)
According to Ponnamperuma, hydrogen cyanide may be "the most important intermediate leading to the origin of life."29 It is an ingredient for the production of amino acids in the Strecker synthesis (see Chapter 3). It also is considered a starting material in the synthesis of adenine and a host of other biomolecules, as shown in figure 4-1. The value of HCN in the chemical evolution scenario is enhanced by the fact that it escapes rapid destruction in the atmosphere by ultraviolet irradiation.30
....
It is the ubiquitous water molecule, however, that is the main obstacle to the reaction involving HCN and its nitrile derivatives.31 For example, HCN adds water to its triple bond to form formamide, which, upon further hydrolysis, produces formic acid."
This is why formic acid, not amino acids. is the primary chemical reaction product in synthesis experiments including Miller-Urey.
This is an example of basic chemistry ignored by chemical evolutionists. Simply: HCN + H2O yields formamide + H2O yields formic acid.
Page 50 goes into more detail as to the destruction of chemical pathways to molecules required for life.
That is for my next post.
Charles Thaxton is affiliated with charlatans at the Disco'tute. Dean Kenyon is a notorious YEC'ist who is credible only among like-minded charlatans.
A typical debate tactic on the incorrect side of a debate is to attack the person reporting the scientific evidence instead of actually addressing the evidence.
I doubt you will actually post about the chemical reaction of HCN with water - but feel free to surprise me!
Oh, and surprise me more by actually confirming the chemical reaction product proportions in origin of life synthesis experiments! Do you deny formic acid rather than amino acids is the predominant product?
Why don't your sources present their work to the journal Nature, for example?
Can you identify where your sources applied their experiments in the conditions likely to have been in place on an early planet?The obvious problem with the book you linked to is the presumption that the authors have a predefined conclusion.T
We can only speculate on what the conditions of the early earth were and what the first life was made of. Here is some on the carbon cycle. I do know that there was plenty of oxygen around, I'm pretty sure it was not free, atmospheric oxygen, at least not for long.
The only evidence for an intelligent chemist is a gap in our knowledge. Such gaps have been getting smaller and smaller as we learn more.
Go ahead and add the early gases which you claim. What happens to the Miller-Urey experiment?
Miller-Urey Experiment
www.millerureyexperiment.com
Thaxton, et al, documented the actual chemical reaction product proportions in this book:
From page 23:
"In 1974, Miller reported the amino acids he had obtained in electrical discharge experiments.8 These are listed in table 3-2."
Reference 8. Miller and Orgel, The Origins of Life on the Earth, p. 84. See also: D. Ring, Y. Wolman, N. Friedmann, and S. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 765; Y. Wolman, W.J. Haverland, and S.L. Miller, 1972. Proc. Nat. Acad. Sci. USA 69, 809; S. Miller, 1955. J. Am. Chem. Soc. 77,2351.
Table3-2. Yields of amino acids obtained from sparking a mixture of CFL, NHl, H20 and H2.
Compound
Glycine Alanine a-Amino-n-butyric acid a-Aminoisobutyric acid Valine Norvaline Isovaline Leucine Isoleucine Alloisoleucine Norleucine tert-Leucine Proline Aspartic acid Glutamic acid Serine Threonine Allothreonine
Yield (14M) Compound Yield (14M)
440 790 270 30 19.5 61 5 11.3 4.8 5.1 6.0 < 0.02 1.5 34 7.7 5.0 0.8 0.8
a:y-Diaminobutyric acid a-Hydroxy-')'-aminobutyric acid Sarcosine N-Ethylglycine N-Propylglycine N-Isopropylglycine N-Methylalanine N-Ethylalanine ,8-Alanine ,8-Amino-n-butyric acid ,8-Amino-isobutyric acid "Y-Aminobutyric acid N-Methyl-,8-alanine N-Ethyl-,8-alanine Pipecolic acid a,,B-Diaminopropionic acid Isoserine
33 74 55 30 2 2 15 < 0.2 18.8 0.3 0.3 2.4 5 2 0.05 6.4 5.5
(From S. Miller, 1974. Origins of Life 5, 139.)
See the link for correct formatting.
Note this list does not include formic acid, the primary product, because it is not an amino acid.
Note Glycine has the highest proportion. This is because Glycine is the simplest amino acid. From Bing search:
"The simplest, and smallest, amino acid found in proteins is glycine for which the R-group is a hydrogen (H)."
There are about 500 naturally occurring amino acids - only 20 (L polarized) are used in life.
Table 3-3 (page 25) includes the primary chemical reaction product: formic acid. It is the base of comparison of chemical reaction product proportions with a number of 1,000. By comparison, Glycine is 270 and Glycolic acid is 240. Some of the amino acids produced are extremely low percentage compared with non-biologic product proportions - see the chart.
See the rest of the evidence of different synthesis experiments in different environments in documented in chapter 3.
Chapter 4 is entitled "The myth of of the prebiotic soup" shows much evidence from chemistry.
I will post just one example in this post. From page 48:[HCN is Hydrogen Cyanide]
"Hydrolysis of HCN and Nitriles (RCN)
According to Ponnamperuma, hydrogen cyanide may be "the most important intermediate leading to the origin of life."29 It is an ingredient for the production of amino acids in the Strecker synthesis (see Chapter 3). It also is considered a starting material in the synthesis of adenine and a host of other biomolecules, as shown in figure 4-1. The value of HCN in the chemical evolution scenario is enhanced by the fact that it escapes rapid destruction in the atmosphere by ultraviolet irradiation.30
....
It is the ubiquitous water molecule, however, that is the main obstacle to the reaction involving HCN and its nitrile derivatives.31 For example, HCN adds water to its triple bond to form formamide, which, upon further hydrolysis, produces formic acid."
This is why formic acid, not amino acids. is the primary chemical reaction product in synthesis experiments including Miller-Urey.
This is an example of basic chemistry ignored by chemical evolutionists. Simply: HCN + H2O yields formamide + H2O yields formic acid.
Page 50 goes into more detail as to the destruction of chemical pathways to molecules required for life.
That is for my next post.
Charles Thaxton is affiliated with charlatans at the Disco'tute. Dean Kenyon is a notorious YEC'ist who is credible only among like-minded charlatans.What are you claiming there is evidence for?OK - from page 49, 50 from the above link:
".... many recent scenarios give HCN a prominent place. Also, a variety of HCN-derived nitriles have been suggested as having an important role as condensing agents in the synthesis of biologically significant polymers.34 Examples of condensing agents include cyanogen, cyanamide, dicyanamide, and cyanoacetylene. Some of these were mentioned in the review of ocean experiments in Chapter 3. The ease with which these cyano compounds enter into reaction with water is, however, a major barrier to their usefulness in synthesis. It is, of course, the ability of these cyano compounds to react with water that makes them attractive candidates as condensing agents. The role of a condensing agent is to remove the water that is split-out or produced as a byproduct in polymer formation. For example, when two amino acids react to form a dipeptide, a water molecule is released. Although dimer formation is thermodynamically unfavorable it can be made favorable simply by removing the water; hence the value of the condensing agent. A water solution, however, is a poor place for a condensing agent to perform its role. The condensing agent simply has no ability to discriminate between water molecules and will react with water from any source. Fig. 4-2 shows a number of the reactions to be expected in the primitive ocean."
Note that I earlier had noted that some amino acids prefer a dry, even with condensing agents, environment. (Others prefer wet, acid, alkaline, hot, cold)
Figure 4-2 shows a number of chemical reaction pathways that result from HCN in the presence of water - reacting with water: (= hydrolysis)
1. HCN to dicyanamide + H20 to cyanourea + H2O to Biuret
2. HCN to cyanomide & carbodiimide + H2O to Urea.
3. HCN to cyanogen +H2O to cyanoformamide + H2O to Ethanediamide.
4. HCN to cyanoacetyline + H2O to cyanoacetaldehyde
5. HCN + NH3 (ammonia) to formamidine + H20 to Formic acid
6. HCN + H2O to formamide + H2O to formic acid
All of these products (I have already noted formic acid) are useless to chemical reaction pathways to amino acids.
I will continue to detail the evidence once someone actually addresses the evidence instead of falsely stating that there is no evidence.
Chemical reaction products - please read my posts more carefully.
Something for the chemical religionists.
Claim CB026:
Miller-Urey type experiments produce toxic chemicals, such as cyanide and formaldehyde, but not amino acids.
Source:
Discovery Institute. 2003. A preliminary analysis of the treatment of evolution in biology textbooks currently being considered for adoption by the Texas State Board of Education. http://www.discovery.org/articleFiles/PDFs/TexasPrelim.pdf, p. 5.
Response:
Ellington, Andrew D. and Matthew Levy. 2003. Gas, discharge, and the Discovery Institute. Reports of the National Center for Science Education 23(3-4): 39-40.
CB026: Toxic chemicals from abiogenesis experiments
www.talkorigins.org
Claim CB026:
Miller-Urey type experiments produce toxic chemicals, such as cyanide and formaldehyde, but not amino acids.
Source:
Discovery Institute. 2003. A preliminary analysis of the treatment of evolution in biology textbooks currently being considered for adoption by the Texas State Board of Education. http://www.discovery.org/articleFiles/PDFs/TexasPrelim.pdf, p. 5.
Response:
- Cyanide and formaldehyde are necessary building blocks for important biochemical compounds, including amino acids (Abelson 1996). They are not toxins in this context.
- Miller-Urey experiments produce amino acids among other chemical compounds (Kawamoto and Akaboshi 1982; Schlesinger and Miller 1983).
- Abelson, P. 1996. Chemical events on the primitive earth. Proceedings of the National Academy of Science USA 55: 1365-1372.
- Kawamoto, K. and M. Akaboshi. 1982. Study on the chemical evolution of low molecular weight compounds in a highly oxidized atmosphere using electric discharges. Origins of Life 12(2): 133-141.
- Schlesinger, G. and S. L. Miller. 1983. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. I. Amino acids. Journal of Molecular Evolution 19(5): 376-382.
Ellington, Andrew D. and Matthew Levy. 2003. Gas, discharge, and the Discovery Institute. Reports of the National Center for Science Education 23(3-4): 39-40.
Which part did I get wrong?The intelligence is in the laws of nature which predestined those things. It’s not an accident that life and intelligence is programmed into the fabric of existence.You think intelligence developed on earth before man? Zero evidence for that I'd say.Well we don’t know this is the first occurrence, right?
Do you really believe the organic micro machines of living organisms are happenstance? Have you seen the animations of the assembly line like machinations of these organic machines?
Yes I do. Simple natural laws can give rise to amazing things. Picture trillions of mindless molecules floating in water, what are the odds that they will form themselves into a regular geometric solid where every one is situated exactly like every other? Happens all the time, they are called crystals. No intelligence required.
You are only partly correct - normal for us humans, btw.
The fine tuned laws and properties of our universe allow for the creation of life and for intelligent life - however these do not evolve by chance.
For example, the precisely fine tuned rate for the expansion of our universe allowed for stars including supernovae to exist - and for supernovae to produce the elements needed for the creation of life.
Also the properties of these elements and complex compounds/molecules of these elements allow them to be arranged as informational rather than simply statistical molecules - for example: informational molecules (which also require translation and messenger molecules (e.g. messenger RNA).
However, information does not occur in molecules by chance - entropy works in the opposite direction - hence the difference between dead molecules and living molecules - at death information decays or leaves so that the functions of life cannot proceed.
For life to come into existence, informational molecules not only need to be created, along with translator molecules - but they need to be in the same place at the same time!
Btw - crystals are repetitive while informational molecules are variant.
The difficulty in creating life (which human creators cannot do) is illustrated in the environments needed to synthesize all of the 20 amino acids required for life:
Some amino acids prefer hot, others prefer cold for synthesis. Some prefer acid, others neutral or alkaline. Some prefer wet, others prefer dry - some even require condensing agents. You cannot have hot & cold, acid and alkaline, wet and dry in the same place at the same time. Unless, of course, an intelligent chemist is involved - of superior intelligence to us humans.
Would you all like me to post details as to the results of synthesis experiments like those of Miller - Urey, etc.? Suffice it to say for now that most are unaware that the primary chemical reaction product is formic acid, not amino acids. And that most amino acids and other molecules produced (the chemical reaction product proportions) are mostly useless (or worse) to life.
And, finally, chance synthesis of polypeptides from these amino acids and then further to proteins are always statistical, not informational. [chance formation of even statistical proteins has an incredibly low probability given favorable primordial soups.]
I should add the need for exact 3-d fit of enzymes and receptors for the life processes to proceed.
Perhaps I should rephrase. Indeed the universe is fine tuned for life as we know it - you are correct about that.
I was pointing out more detail. The fine tuning of our universe allowed for the creation of life as we know it. However, life does not spontaneously form from the elements that supernovae produce - see my posts for more detail. Life still needed an intelligent creator so as to produce the needed informational molecules at the same place and time.
An example is HCN (1 atom Hydrogen, 1 atom carbon, 1 atom Nitrogen). Hydrogen was produced without stars, supernovae produced carbon, nitrogen and oxygen (the O in H2O). But the reactions of HCN with H2O do not lead to molecules required for life in significant proportions. An intelligent chemist is needed to isolate/select each step towards simple and complex amino acids and to biologically important dipeptides to polypeptide to proteins.
The issue has never been one of chemical chains that resemble proteins. The issue has always been about folding instructions.
Ah, laundry at last! Want me to fold?
Seriously:
Well, there are many issues. 3-d folding is more complex than chemical reactions - feel free to post on that - e.g. the specific 3-d fit between enzymes and receptors.
Similar threads
- Replies
- 136
- Views
- 564
- Replies
- 210
- Views
- 1K
- Replies
- 311
- Views
- 3K
Latest Discussions
- Replies
- 1K
- Views
- 26K
- Replies
- 167
- Views
- 809
Forum List
-
-
-
-
-
Political Satire 8521
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
ObamaCare 781
-
-
-
-
-
-
-
-
-
-
-
Member Usernotes 484
-
-
-
-
-
-
-
-
-
-