Toddsterpatriot
Diamond Member
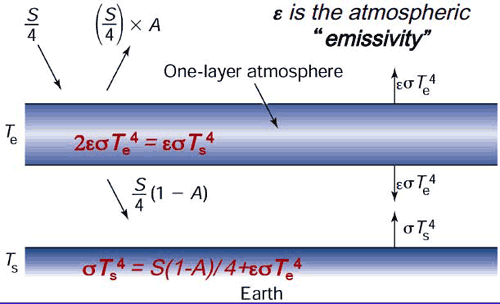
P= zero. yep!! no power.The one we disagree on is the one were t can equal tc and zero is the answer.hey dude, I stated many times, I don't have any qualms with that equation. none. The one we disagree on is the one were t can equal tc and zero is the answer.post the piece that shows energy flowing from cool to hot. I've been waiting. you fail every day.Right, no evidence behind Stefan-Boltzmann. DURR
View attachment 284593
Plug in your variables. This formula will show you the power radiated by the cool object.
Net is zero. Why do you disagree?
Yup, net=0