mamooth
Diamond Member
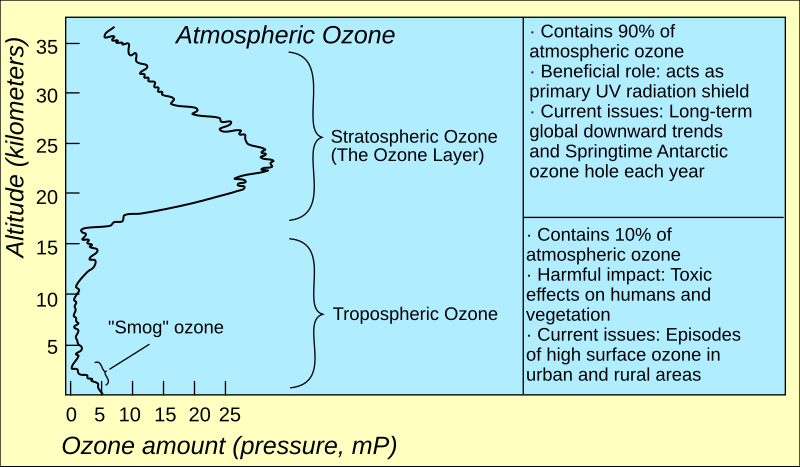
Use your brain for just a moment....if ozone has the long half life you claim, why is it not a well mixed gas in the atmosphere?
We'll try to start with the basics. Can you tell us why "the stratosphere" is called "the stratosphere"? I just ask because you don't seem aware of how the stratosphere works.