SSDD
Gold Member
- Nov 6, 2012
- 16,672
- 1,966
- 280
- Thread starter
- #1,181
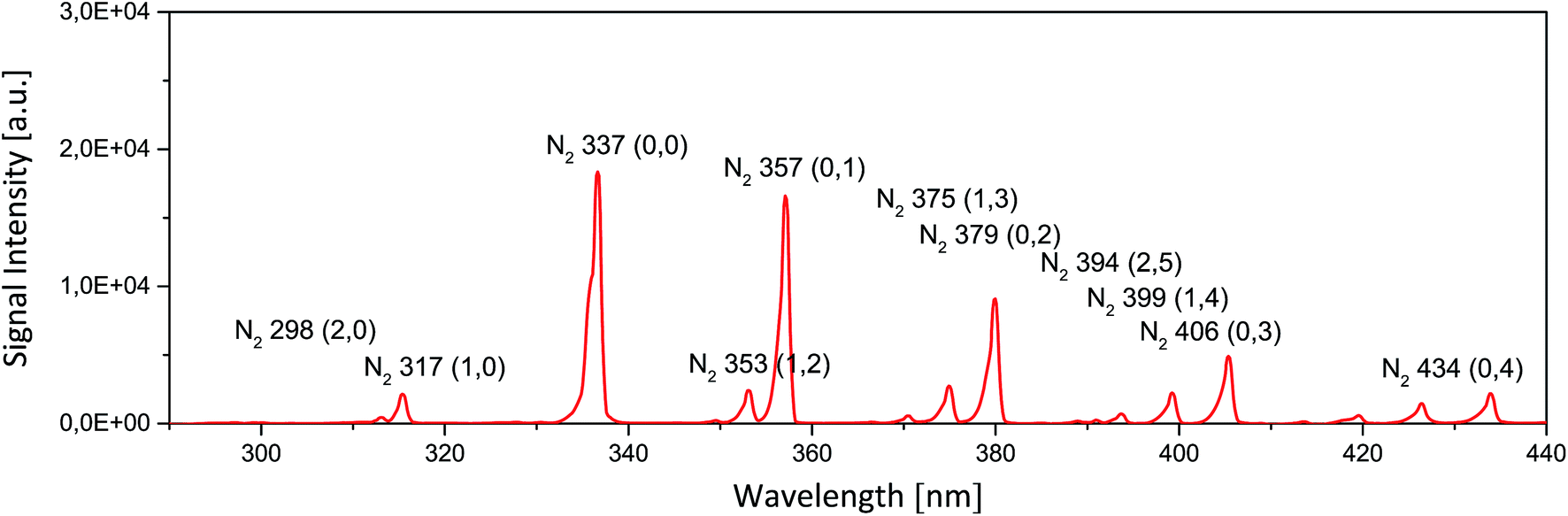
So now you've flipped? CO2 loses its energy by radiation?
Did you think no one would notice the change?
Can you point to any where that I have said that CO2 never radiates energy it absorbs? Anywhere at all? Keep up skid mark...is imagining what I have said really the best you can do?